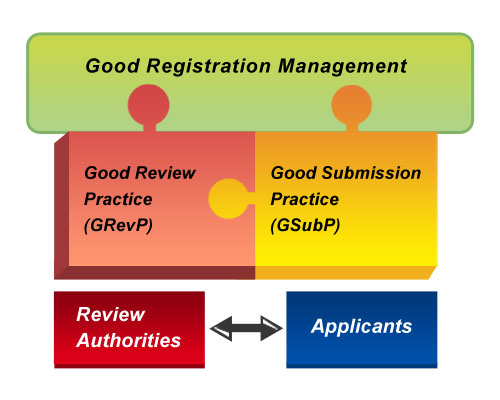
GRM STEERING COMMITTEE
2018 GRM Steering Committee Membership List
| Economy | Name | Affiliation |
| Chinese Taipei | Jo-Feng Chi
Churn-Shiouh Gau
Rosa Fu |
TFDA
CDE
IRPMA |
| Japan | Eriko Fukuda
Shinji Hatakeyama |
PMDA
JPMA |
| Singapore | Silke Vogel | CoRE, Duke-NUS |
| United States | Paul Brooks
Lawrence Liberti |
RAPS
CIRS |
2020 GRM Steering Committee Membership List
| Economy | Name | Affiliation | GRM Steering Committee Role |
| Chinese Taipei | Chyn-Liang Huang
Mei-Chen Huang
Finny Liu (劉瑞芬) |
Taiwan FDA
Taiwan FDA
IRPMA |
Co-Chair
Member
Member |
| Japan | Daisuke Koga
Hirooki Tanabe
Shinji Hatakeyama |
PMDA
MHLW
APAC |
Co-Chair
Member
Member |
| Thailand | Suchart Chongprasert | Thai FDA | Member |
| United States | Lawrence Liberti
Min Chen (李敏珠顧問) |
CIRS
Retired from US FDA (2014) |
Member
Member |
2022 GRM Steering Committee Membership List
| Economy | Name | Affiliation | GRM Steering Committee Role |
| Chinese Taipei | Chyn-Liang Huang
Chia-Ping Liu
Finny Liu (劉瑞芬) |
Taiwan FDA
Taiwan FDA
IRPMA |
Co-Chair
Member
Member |
| Japan | Junko Sato
Mao Yanagisawa
Shinji Hatakeyama |
PMDA
MHLW
APAC |
Co-Chair
Member
Member |
| Thailand | Suchart Chongprasert | Thai FDA | Member |
| United States | Lawrence Liberti
Min Chen (李敏珠顧問) |
Temple University School of Pharmacy
Retired from US FDA (2014) |
Member
Member |
2023 GRM Steering Committee Membership List
| Economy | Name | Affiliation | GRM Steering Committee Role |
| Chinese Taipei | Shirley Pan
Kuo-Teng Hung
Finny Liu (劉瑞芬) |
Taiwan FDA
Taiwan FDA
IRPMA |
Co-Chair
Member
Member |
| Japan | Ayumi Endo
Yuriko Takemura
Mao Yanagisawa
Shinji Hatakeyama |
PMDA
PMDA
MHLW
APAC |
Co-Chair
Member
Member
Member |
| Chinese Taipei | Worasuda Yoongthong
Nantana Kaisaeng
Wimolsiri Punjatanasak |
Thai FDA
Thai FDA
APAC (Thailand) |
Member
Member
Member |
| United States | Lawrence Liberti
Min Chen (李敏珠顧問) |
USC
Retired from US FDA (2014) |
Member
Member |